Pembrolizumab doubles time to disease progression in patients with advanced colorectal cancer with specific DNA mutations
American Society of Clinical Oncology 2020 Annual Meeting Press Release May 30, 2020
Front-line therapy with the immune checkpoint inhibitor pembrolizumab (Keytruda) doubled progression-free survival vs chemotherapy in patients with a type of advanced colorectal cancer that has a high number of mutations, which previous research suggests may have a poor prognosis for some patients. This is the first time pembrolizumab has been shown to benefit these patients when used as a front-line therapy. The findings come from an interim analysis of the phase III KEYNOTE-177 trial that will be presented during the virtual scientific program of the 2020 American Society of Clinical Oncology (ASCO) Annual Meeting.

ASCO Perspective
“Immunotherapies like pembrolizumab have proved to be effective as second-line treatments for advanced disease. Now, in studies like this one, we are starting to see significant efficacy for immunotherapies as first-line treatment for advanced cancers with specific genetic signatures, in this case metastatic colorectal carcinoma with microsatellite instability high/mismatch repair deficient mutations. The data presented have the potential to change the standard of care,” said ASCO President Howard A. Burris III, MD, FACP, FASCO.
Study at a Glance
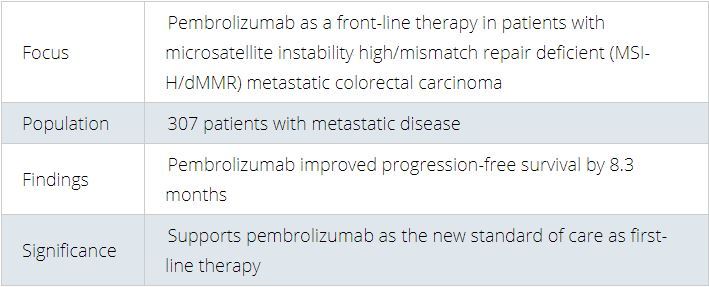
Progression-free survival with first-line pembrolizumab was 16.5 months compared with 8.2 months with chemotherapy with or without targeted therapy, establishing pembrolizumab as the new standard of care for patients with microsatellite instability high/mismatch repair deficient (MSI-H/dMMR) metastatic colorectal carcinoma.
“These long-awaited trial results will change clinical practice,” said lead author Thierry André, MD, of the Sorbonne Université and Hôpital Saint Antoine in Paris. “Pembrolizumab works in non-randomized studies in this group of patients with advanced disease. This randomized study demonstrates a huge benefit in first line with pembrolizumab and should be the new standard of care.”
Approximately 5% of patients with metastatic colorectal cancer have high microsatellite instability, which is the presence of high levels of mutations. In MSI-H/dMMR, DNA repair is impaired, resulting in an increased number of mutations. For some patients previous research suggests that the presence of MSI-H/dMMR tumors is associated with decreased survival, and patients with MSI-H/dMMR metastatic disease are less responsive to conventional chemotherapy.
Previous research has shown good response to pembrolizumab and longer survival for MSI-H metastatic colorectal cancer, refractory to chemotherapy. Pembrolizumab blocks the activity of a receptor called PD-1, a protein that helps keep the immune system in check, thereby allowing the immune system to attack cancer cells.
Key Findings
At 12- and 24-months follow up, progression-free survival was 55.3% and 48.3% with pembrolizumab vs. 37.3% and 18.6% with chemotherapy, respectively. The proportion of patients with a reduction in tumor size (objective responsive rate) was better with pembrolizumab as well — 43.8% compared with 33.1% for chemotherapy. Eleven percent of patients receiving pembrolizumab had complete response (no detectable cancer); 32.7% had a reduction in tumor size (partial response); and 30.9% had stable disease. In comparison, 3.9%, 29.2% and 42.2% of patients receiving chemotherapy had complete response, partial response, and stable disease, respectively. Response with pembrolizumab was also longer lasting, with 83% of patients having a response longer than 2 years, compared with 35% of patients receiving chemotherapy.
Severe treatment-related adverse events (grade 3 or greater) were also less common with pembrolizumab than chemotherapy (22% vs. 66%). The profile of toxicities is very different between both groups with immune-mediated adverse events with pembrolizumab (colitis and hepatitis) and classical most frequent chemotherapy toxicities for the chemotherapy arm with diarrhea, neutropenia, fatigue, nausea and vomiting, stomatis, alopecia, and neurotoxicity.
About the Study
At the time of data cutoff date for this interim analysis (February 19, 2020), the study included 307 patients with MSI-H/dMMR mCRC. Patients were randomly assigned to receive first-line pembrolizumab for up to 2 years or the investigator’s choice of six different standard chemotherapy regimens, selected prior to randomization. The investigators could choose from mFOLFOX6 (5-fluorouracil, leucovorin, and oxaliplatin); mFOLFOX6+bevacizumab; mFOLFOX6+cetuximab; FOLFIRI (leucovorin, 5-fluorouracil, and irinotecan); FOLFIRI+bevacizumab; or FOLFIRI+cetuximab.
PFS and overall survival were the primary end points. Key secondary end points included overall response rate and safety.
Next Steps
Patients were allowed to cross over at progression from the chemotherapy group to the pembrolizumab group. The study will continue to evaluate overall survival.
This article is a news release from American Society of Clinical Oncology 2020 Press Meeting. Read the original here.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries