Phage Therapy vs. Superbugs: A Post-Antibiotic Era Solution
M3 India Newsdesk Jun 11, 2025
Phage therapy harnesses viruses that target bacteria, offering a promising solution to combat antibiotic-resistant superbugs. This article explains how phage therapy offers a targeted and innovative approach to treating antibiotic-resistant bacterial infections.

The rise of antibiotic-resistant bacteria, or "superbugs," poses a global health crisis, rendering conventional antibiotics ineffective and threatening modern medicine. As we approach a post-antibiotic era, phage therapy—the use of bacteriophages (viruses that infect bacteria) to combat bacterial infections—has re-emerged as a promising alternative. This article examines the mechanisms, advantages, and challenges of phage therapy in comparison to traditional antibiotics.
We review recent clinical successes, such as the use of phages against multidrug-resistant Pseudomonas aeruginosa and Staphylococcus aureus, and discuss advancements in phage engineering and synthetic biology that enhance therapeutic efficacy. Despite regulatory and logistical hurdles, phage therapy offers a targeted, adaptable, and sustainable solution to antimicrobial resistance (AMR). This paper highlights the potential of phage therapy to revolutionise infection treatment and restore hope in the fight against superbugs.
Introduction
Antimicrobial resistance (AMR) is projected to cause 10 million deaths annually by 2050, surpassing cancer as a leading cause of mortality (O’Neill, 2016) [1]. The overuse and misuse of antibiotics have accelerated the evolution of resistant pathogens, including methicillin-resistant Staphylococcus aureus(MRSA), carbapenem-resistant Enterobacteriaceae (CRE), and extensively drug-resistant Mycobacterium tuberculosis(XDR-TB). With dwindling antibiotic pipelines, alternative treatments are urgently needed.
Phage therapy, first discovered in 1917 by Félix d’Hérelle, offers a viable solution. Bacteriophages (phages) are natural predators of bacteria, capable of lysing antibiotic-resistant strains with precision.
Unlike broad-spectrum antibiotics, phages are strain-specific, minimising collateral damage to beneficial microbiota. Recent advances in genomics and synthetic biology have revitalised phage therapy, making it a key contender in the post-antibiotic era.
This article explores:
- The mechanisms of phage therapy and its advantages over antibiotics.
- Clinical applications in treating multidrug-resistant infections.
- Challenges, including regulatory barriers and phage resistance.
- Prospects, including engineered phages and combination therapies.
Mechanisms of Phage Therapy
How Phages Kill Bacteria
Phages infect bacteria through a lytic cycle:
- Attachment: Phages bind to specific bacterial surface receptors.
- Genome Injection: Viral DNA/RNA enters the host.
- Replication & Assembly: The host machinery produces new phage particles.
- Lysis: Enzymes like endolysins rupture the bacterial cell wall, releasing progeny phages.
Lytic phages (e.g., T4 phage) are preferred for therapy, while temperate phages (which integrate into the host genome) are avoided due to potential horizontal gene transfer.
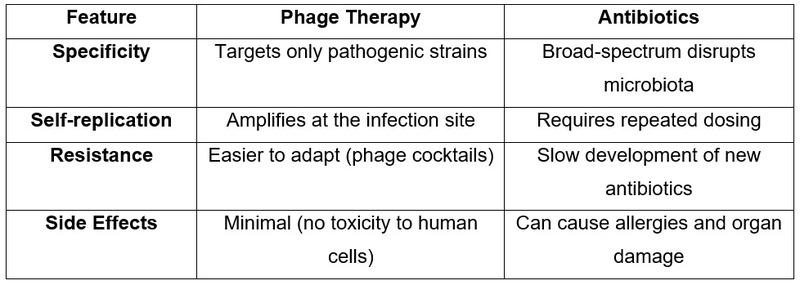
Advantages Over Antibiotics
Clinical Successes of Phage Therapy
Case Studies
- 2016 (USA): A patient with Acinetobacter baumannii infection was successfully treated with a phage cocktail after failing antibiotic therapy [2].
- 2019 (UK): A teenage cystic fibrosis patient with Pseudomonas aeruginosa infection showed lung function improvement after phage therapy [3].
- 2020 (Belgium): Personalised phage therapy cured a chronic MRSA prosthetic joint infection [4].
Ongoing Trials
- Phagoburn (EU): Testing phages against E. coli and P. aeruginosa burn infections.
- Adaptive Phage Therapeutics (USA): Developing phage banks for military wound infections.
Challenges and Limitations
Regulatory Hurdles
-
Phages are classified as drugs in the U.S. (FDA) and biological products in the EU (EMA), requiring lengthy approvals.
- Lack of standardised protocols complicates clinical adoption.
Bacterial Resistance to Phages
- Bacteria evolve CRISPR-Cas and restriction-modification systems to evade phages.
- Solution: Use phage cocktails or engineer CRISPR-phages.
Manufacturing & Storage
- Phages require cold storage and host-specific cultivation.
- Solution: Synthetic biology enables stable, shelf-ready phage formulations.
The Future of Phage Therapy
Engineered Phages
- CRISPR-phages: Deliver bacterial-killing genes (e.g., targeting antibiotic resistance plasmids).
- Phage-antibiotic synergy (PAS): Combining phages with antibiotics enhances efficacy.
Personalised Phage Medicine
- AI-driven phage matching from global databases (e.g., PhageDB).
- On-demand phage production using microfluidic platforms.
Global Phage Banks
The Eliava Institute (Georgia) and Phage Directory (USA) curate phage libraries for rapid deployment.
Conclusion
Phage therapy represents a paradigm shift in combating superbugs, offering precision, adaptability, and sustainability absent in antibiotics. While challenges in regulation, resistance, and scalability persist, advances in synthetic biology, AI, and global collaboration are accelerating clinical translation. As antibiotic resistance escalates, phage therapy may soon transition from a last-resort option to a first-line defence, securing its place as a cornerstone of post-antibiotic medicine.
Disclaimer: The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of M3 India.
About the author of this article: Dr Partha Ghosh, BNYS, MD(YS), is a general physician and a medical writer from Siliguri, Darjeeling.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries