Article: Clinical Guide to Molecular Testing in Bethesda III and IV Nodules for Indian Practice
M3 India Newsdesk May 09, 2025
Molecular diagnostics have revolutionised Bethesda III and IV nodules management, providing clarity in otherwise ambiguous cases. This article explains the role of molecular testing in the management of Bethesda III and IV nodules, along with the practical recommendations for Indian clinicians.
Background
In clinical practice, thyroid nodules are very prevalent, particularly in iodine-rich areas. While the majority are benign, about 5–15% prove malignant. FNAC (fine-needle aspiration cytology) is the gold standard first-line investigation. However, 20–25% of thyroid FNAS fall into the indeterminate Bethesda categories III (AUS/FLUS) or IV (FN/SFN), where the risk of malignancy (ROM) ranges from 10–40%.
The main clinical dilemma: most of these nodules are benign, yet many patients undergo diagnostic surgery(lobectomy) for clarification. This is where molecular testing now plays a transformative role in stratifying risk and avoiding unnecessary surgery.
Bethesda III/IV Cytology: Definitions and Risk
The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) classifies thyroid FNA results into six categories. Bethesda III – also termed Atypia of Undetermined Significance (AUS) or Follicular Lesion of Undetermined Significance (FLUS) – indicates that cells show minor atypia or changes that are not benign or malignant.
Bethesda IV – Follicular Neoplasm or Suspicious for Follicular Neoplasm (FN/SFN) – indicates a predominantly follicular cell pattern that raises suspicion for follicular thyroid carcinoma (including Hürthle cell neoplasms) but isn’t diagnostic without surgical histology. In practice, these indeterminate diagnoses are made when cytologic features are equivocal. For example, a sparsely cellular sample with focal nuclear atypia might be labelled AUS, or a cellular sample with a uniform microfollicular pattern may be labelled as FN/SFN.
Clinicians should suspect an indeterminate result when the cytopathologist’s report mentions atypia or a possible follicular neoplasm without a clear malignant diagnosis. The risk of malignancy (ROM) for Bethesda III and IV nodules is intermediate between benign and overtly suspicious categories.
In the past, Bethesda III nodules typically had between 10 and 30 per cent ROM, while Bethesda IV nodules carried between 25 and 40 per cent. They are very prevalent in clinical practice, particularly in iodine-rich areas. (The exact risk varies by institution and whether noninvasive follicular thyroid neoplasm with papillary-like nuclei (NIFTP) is counted as “malignant.”
Reclassification of NIFTP as a benign tumour has slightly lowered the observed ROM in these categories by a few.) In any case, an indeterminate cytology result means a significant minority of these nodules will turn out to be thyroid cancers on final histopathology, while the majority are benign. This uncertainty is what makes management challenging.

Conventional Management Pathways
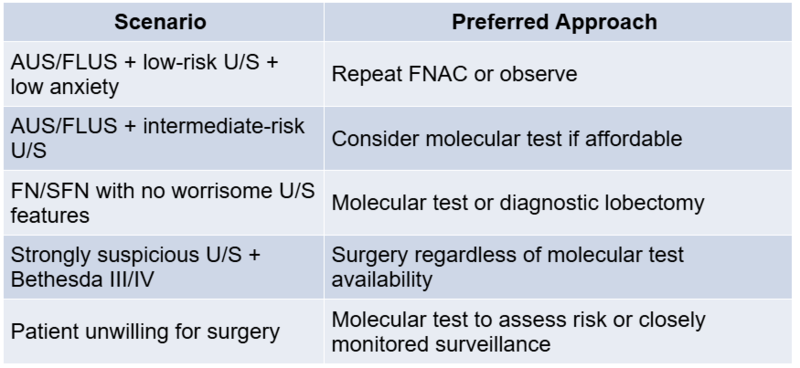
A. Repeat FNAC:
- Preferred in Bethesda III lesions
- Performed under ultrasound guidance
- May be reclassified into benign or suspicious categories
B. Ultrasound Stratification:
- Use ATA patterns or ACR TI-RADS
- Suspicious features (e.g., irregular margins, microcalcifications) increase the likelihood of malignancy
C. Diagnostic Lobectomy:
- Common for Bethesda IV or persistently indeterminate nodules
- Therapeutic if malignant, but often reveals benign histology
D. Active Surveillance:
- Feasible for <1.5 cm, low-suspicion nodules
- Requires reliable follow-up and patient counselling
Role of Molecular Testing
Molecular testing aids in further risk stratification of indeterminate nodules and is used in two main ways:
Common Molecular Targets:
- Mutations: BRAF V600E, RAS, TERT, TP53
- Gene Fusions: RET/PTC, NTRK, PAX8/PPARG
- Expression Profiles: mrna, microRNA panels
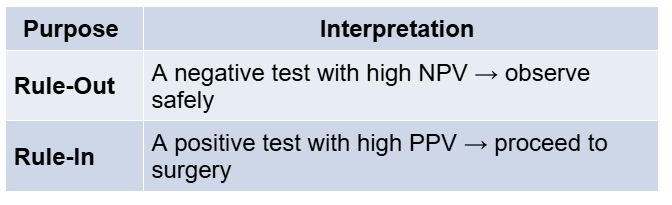
Molecular testing refers to analysing the FNA sample for genetic alterations or gene expression profiles associated with thyroid cancer. The goal is to improve risk assessment beyond what cytology alone can provide. In practical terms, a good molecular test can either rule out malignancy in a nodule (if the test result is “benign” or “negative”) with high confidence, allowing the patient to avoid surgery, or rule in malignancy (if the test is “suspicious” or “positive”) by detecting mutations or patterns that strongly indicate cancer, thereby guiding the clinician to proceed with appropriate surgery.
These tests target the molecular drivers of thyroid cancer. Thyroid carcinogenesis commonly involves certain genetic pathways – for example, the MAPK and PI3K-AKT signalling pathways are frequently altered. Point mutations in genes like BRAF and RAS, and gene fusions/rearrangements such as RET/PTC, NTRK, or PAX8/PPARG, are well-known in thyroid cancers. For instance, the BRAFV600E mutation (a constitutively active BRAF kinase) is present in roughly 60–70% of papillary thyroid carcinomas and strongly predicts malignancy. RAS mutations are found in a significant subset of indeterminate nodules and can indicate follicular variant PTC or follicular carcinoma (though RAS-mutant nodules can also be indolent adenomas).
Other alterations, like TERT promoter mutations or p53, occur in more aggressive tumours but are less common in indeterminate cytology. Besides DNA mutations, differences in mrna expression patterns and microrna profiles between benign and malignant nodules can be harnessed for diagnosis.
By detecting these molecular signatures in FNA material, we can refine the estimated probability of cancer. A “negative” molecular test (no high-risk mutations or a benign gene expression profile) generally implies the nodule is likely benign, often >94–95% chance of being benign. This high negative predictive value (NPV) allows such patients to be followed with observation instead of surgery. On the other hand, a “positive” or “suspicious” molecular test (finding a mutation or a genomic profile suggestive of cancer) indicates a higher likelihood of malignancy, sometimes in the 50–75% range, supporting the need for. In some cases, knowing the specific mutation can also guide the extent of surgery (e.g., a nodule with BRAFV600E – which essentially confirms papillary carcinoma – might prompt a total thyroidectomy and lymph node evaluation in a young patient, whereas a RAS mutation might be handled with an initial lobectomy given its association with lower-grade tumors).
Molecular testing has been recognised in guidelines as a useful adjunct for Bethesda III/IV nodules. The ATA (2015) and other societies stopped short of recommending any particular test, but they acknowledge that molecular marker testing can complement cytology to guide. It is particularly valuable in settings where a decision is equivocal: for example, a patient with an AUS nodule who strongly wishes to avoid surgery might safely do so if a high-quality molecular test comes back benign.
Conversely, a patient with indeterminate cytology and a positive molecular test can be counselled that surgery is likely the appropriate next step. Importantly, these tests are ancillary tools– they do not diagnose cancer with absolute certainty, but they shift the probability. A very small risk of false-negative results remains, so clinical judgement and patient counselling are still crucial (for instance, continued ultrasound surveillance is recommended even for molecularly “benign” nodules).
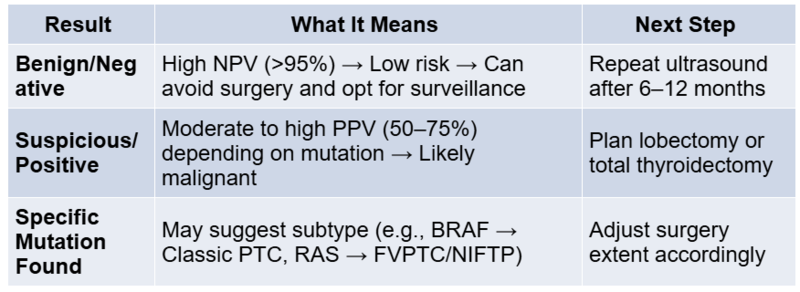
How to Collect a Sample for Molecular Testing
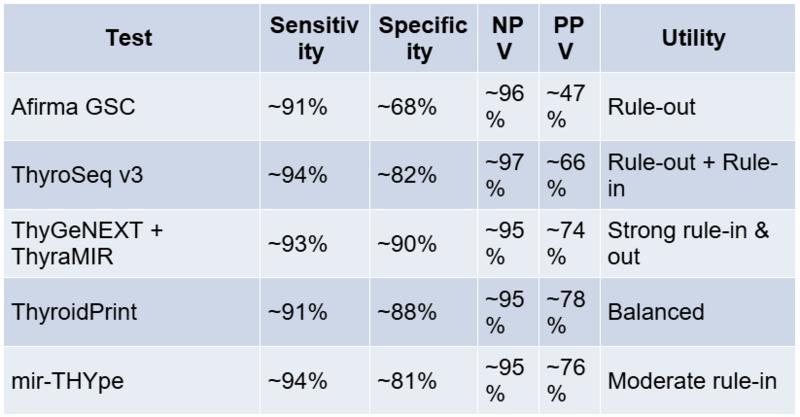
Afirma testing requires dedicated FNA passes to be collected in a proprietary RNA preservation solution (the test kit). Typically, 2–3 passes from the nodule are procured after the diagnostic smears. The sample is shipped to Veracyte’s laboratory in the US for analysis. Turnaround time is about 1–2 weeks from sample receipt. Advantages: Afirma has been validated in multicenter studies and has a high NPV, covering a broad array of gene expression signals (it can even flag potential medullary carcinoma or parathyroid tissue). Limitations: A suspicious result is relatively non-specific (many false positives), so it’s mainly useful for ruling out disease. Additionally, the test is expensive, and processing is centralised (for Indian patients, this means logistical challenges in sending samples abroad).
Interpretation of Test Results
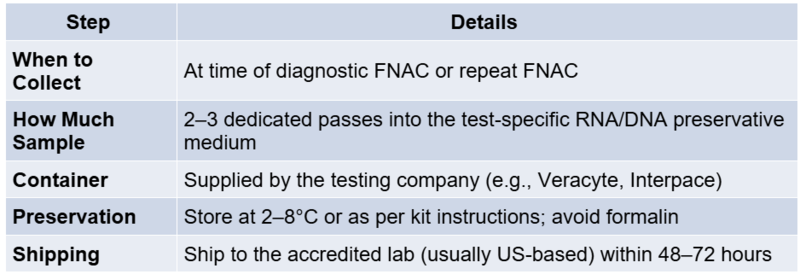
When a molecular test yields a positive result, interpretation depends on the specific alteration detected. Each mutation or fusion carries distinct implications:
- BRAF V600E: This mutation is highly specific for classic papillary thyroid carcinoma (PTC). Its presence virtually confirms malignancy. Clinicians should consider total thyroidectomy and evaluate for central neck lymph node dissection, especially in younger patients or those with high-risk features.
- NRAS/HRAS/KRAS mutations: These are commonly found in follicular-patterned lesions. While associated with follicular carcinoma and follicular variant PTC, they can also be present in benign adenomas or NIFTP. A lobectomy is often sufficient for diagnosis and treatment unless other high-risk factors exist.
- TERT promoter mutations or TP53: These alterations are linked to aggressive behaviour and poor prognosis, especially when co-existing with BRAF. Consider total thyroidectomy and closer surveillance or adjuvant therapy.
- RET/PTC, NTRK, ALK fusions: These gene rearrangements are associated with PTC and more invasive subtypes, sometimes seen in radiation-associated cancers. They may qualify patients for targeted therapies in advanced disease.
Each result should be interpreted within the clinical, cytological, and radiological context to avoid over- or under-treatment.
Turnaround Time
Comparative Performance Table
Indian Context: Practical Challenges
Cost
- Molecular tests cost ₹2–2.5 lakh (~USD 3000+)
- Diagnostic lobectomy in India: ₹50,000–₹1,00,000
Logistics
- Samples need to be shipped abroad (cold chain, customs clearance)
- Delays in report receipt
Insurance
- Most public and private insurers in India do not cover molecular testing
Current Indian Alternatives
- Repeat FNAC
- Ultrasound monitoring
- Surgery
- Pilot Indian molecular panels under development at select centres (AIIMS, TMH, etc.)
Practical Recommendations for Indian Clinicians
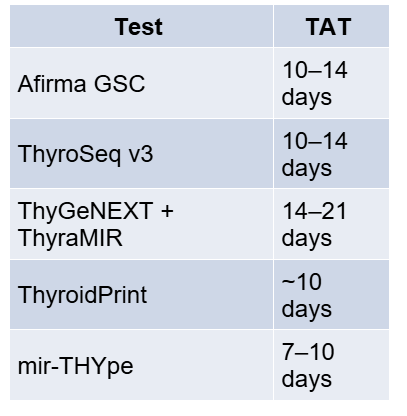
While molecular testing has become routine in many Western centres, its availability in India and other South Asian countries remains extremely limited in 2025. Currently, the well-known commercial tests (Afirma, ThyroSeq, ThyGeNEXT/Thyramir, etc.) are not readily offered in most Indian hospitals due to a combination of cost and logistical constraints.
The tests have to be sent to specialised labs (often in the US), incurring very high costs. In India, the estimated cost of such molecular panels is at least USD 3000 (roughly ₹2–2.5 lakh) per test, which is many times the cost of a thyroid surgeryBy comparison, the cost of a hemithyroidectomy in an average private Indian hospital might be on the order of ₹50,000–₹1,00,000, and even lower in government centers.
Thus, from a purely economic standpoint, performing a diagnostic surgery can often be cheaper than the test! Furthermore, thyroidectomy, while invasive, is widely available, whereas these molecular assays are not yet established in Indian labs.
Beyond cost, there are infrastructure and regulatory hurdles – maintaining sample integrity during international shipping, coordinating with overseas labs, and uncertainties in insurance coverage. For these reasons, molecular testing is currently seldom used in routine practice in India for indeterminate thyroid nodules. Most Indian clinicians still manage Bethesda III/IV nodules with a combination of repeat FNAC, ultrasound monitoring, and upfront surgery when indicated.
However, the high prevalence of thyroid nodules and the desire to avoid unnecessary surgeries in India have spurred interest in developing low-cost local testing solutions.
Researchers in India have been exploring simplified mutation panels that could be run domestically at a lower cost. For example, a pilot study in 2022 described a laboratory-developed NGS panel tailored for indeterminate FNAS, aiming to detect common mutations cost-effectively.
The authors argued that given the cost and quality-of-life implications of thyroidectomy, a locally available, affordable molecular test could be very valuable in the Indian setting. Such a test might not need to cover as many genes as ThyroSeq, but if it can reliably detect the most common actionable mutations (like BRAF, RAS, RET/PTC, PAX8-PPARG, etc.) at a fraction of the cost, it could significantly aid decision-making. Another consideration is population-specific validation – the mutation prevalence in Indian patients (e.g., the rate of RAS mutations or NIFTP incidence) may differ slightly from Western cohorts, so any indigenous panel should be validated on local data.
At present, cost remains the main barrier. The few patients in India who do get molecular testing likely have to pay out-of-pocket and have their samples sent abroad via a speciality referral. As a result, the use of molecular testing in indeterminate thyroid nodules is not standard in India yet. Clinicians, therefore, often proceed with surgery if clinical suspicion is moderate to high, or carefully observe the nodule (with or without a repeat FNAC) if suspicion is low, in the absence of molecular data.
Conclusion
While the cost and logistical barriers currently limit widespread adoption of the management of Bethesda III and IV nodules in India, awareness is essential, particularly for oncologists, endocrinologists, and surgeons dealing with high volumes of thyroid pathology.
By selecting appropriate patients and interpreting molecular test results in the correct clinical context, unnecessary surgeries can be avoided, and more accurate, individualised care can be provided. The future lies in India-specific, low-cost, validated molecular panels that can democratise access and truly bridge the care gap.
Disclaimer- The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of M3 India.
About the author of this article: Dr. Bhavin Vadodaria, MBBS, MD (General Surgery), DNB (Surgical Oncology), is a Head & Neck Surgeon & Consultant Surgical Oncologist at SSO Cancer Hospital, Ahmedabad.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries
